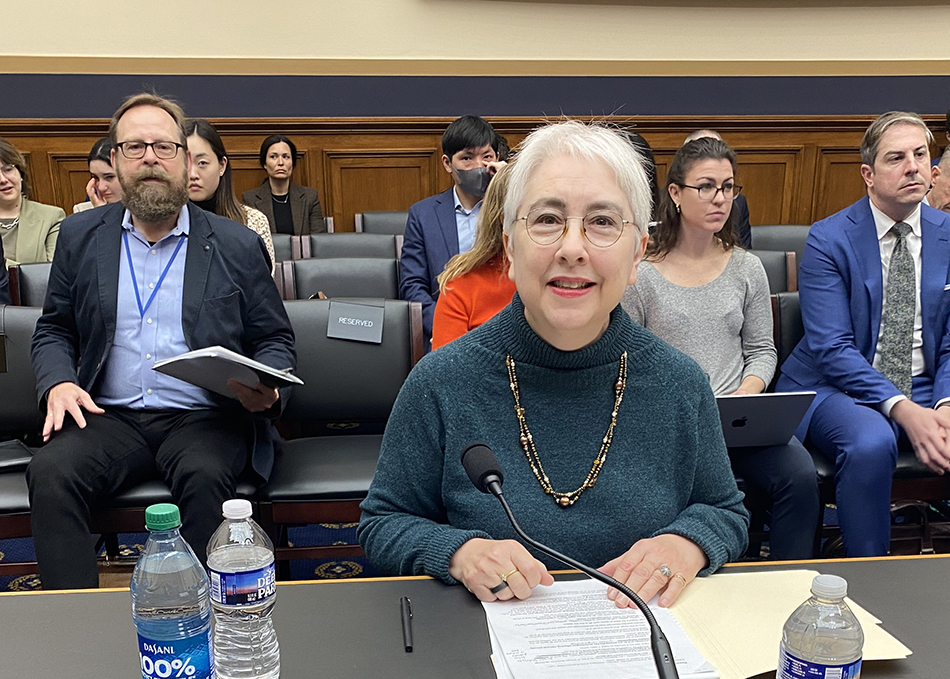
John F. Crowley: ‘Biotechnology is another word for hope’
On April 18, 2024, the Biotechnology Innovation Organization (BIO) held the inaugural Agriculture ...
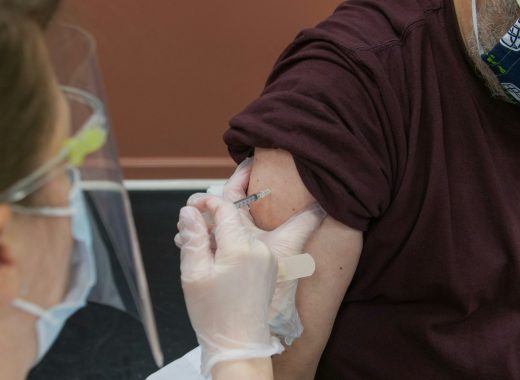
Read More Social value of vaccines far exceeds their cost, new study finds
A recent study conducted by the Office of Health Economics (OHE, the world’s ...
Read More 5 things to know for Primary Immunodeficiency Month
April is Primary Immunodeficiency Month, and in observation, Bio.News is partnering with the ...
Read More Life Sciences PA recognizes John F. Crowley for biotech leadership
On April 10, Life Sciences PA awarded Biotechnology Innovation Organization (BIO) President & ...
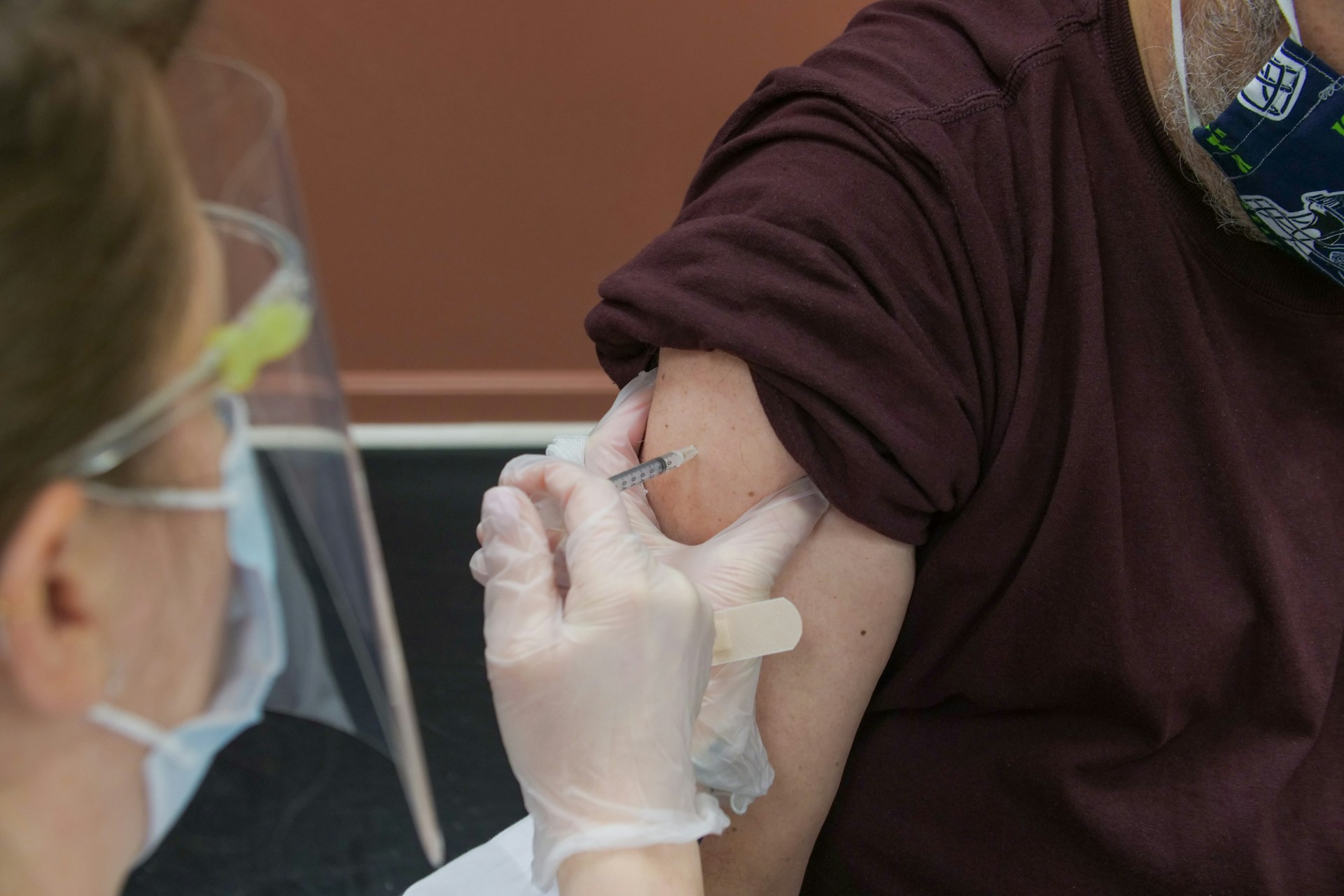
Read More Biotech and One Health are key to controlling avian flu
A recent human infection of avian flu in Texas, coming just days after ...
Read More Biotech is climate tech: how biotech is building a sustainable future
Biotechnology plays a pivotal role when it comes to mitigating climate change and ...
Read More EDITORS' CHOICE
Biotech and One Health are key to controlling avian flu
A recent human infection of avian flu in Texas, coming just days after the first infections of U.S. livestock, has spurred research into the latest ...
April 15, 2024
Read More LATEST NEWS
Agriculture
John F. Crowley: ‘Biotechnology is another word for hope’
On April 18, 2024, the Biotechnology Innovation Organization (BIO) held the inaugural Agriculture & Environment Summit in Washington, D.C., bringing together government and industry leaders ...
April 18, 2024
Health
Social value of vaccines far exceeds their cost, new study finds
A recent study conducted by the Office of Health Economics (OHE, the world’s oldest independent health economics research organization), and funded by the International Federation ...
April 18, 2024
Health
5 things to know for Primary Immunodeficiency Month
April is Primary Immunodeficiency Month, and in observation, Bio.News is partnering with the Immune Deficiency Foundation (IDF) to talk about the condition and what you ...
April 16, 2024
BIO's View
Life Sciences PA recognizes John F. Crowley for biotech leadership
On April 10, Life Sciences PA awarded Biotechnology Innovation Organization (BIO) President & CEO John F. Crowley the Hubert J.P. Schoemaker Leadership Award. The award ...
April 15, 2024
Agriculture
Biotech and One Health are key to controlling avian flu
A recent human infection of avian flu in Texas, coming just days after the first infections of U.S. livestock, has spurred research into the latest ...
April 15, 2024
HEALTH
5 things to know for Primary Immunodeficiency Month
April 16, 2024
Biotech and One Health are key to controlling avian flu
April 15, 2024
Welcome, John F. Crowley!
Get to know John F. Crowley, the new President and CEO of the Biotechnology Innovation Organization (BIO), in our exclusive interview.
AGRICULTURE
John F. Crowley: ‘Biotechnology is another word for hope’
April 18, 2024
Biotech and One Health are key to controlling avian flu
April 15, 2024
How biotech is improving the health of our pets
March 28, 2024
Climate Change
John F. Crowley: ‘Biotechnology is another word for hope’
April 18, 2024
On April 18, 2024, the Biotechnology Innovation Organization (BIO) held the inaugural Agriculture & Environment Summit in Washington, D.C., bringing ...
Read More International Day of Forests: A call for more innovation
March 21, 2024
New Mexico legislature passes clean fuel standard
February 19, 2024
Federal Policy
Heart disease patients and the IRA’s misguided incentives
March 25, 2024
State Policy
Life Sciences PA recognizes John F. Crowley for biotech leadership
April 15, 2024
On April 10, Life Sciences PA awarded Biotechnology Innovation Organization (BIO) President & CEO John F. Crowley the Hubert J.P. ...
Read More Colorado PDAB could limit patient access to medicines
March 20, 2024
New Mexico legislature passes clean fuel standard
February 19, 2024
International
5 reasons for investor optimism at BIO-Europe Spring 2024
March 29, 2024
WTO Ministerial ends, no expansion of COVID IP waiver
March 4, 2024
BIO Board member tells Congress WTO IP waivers hurt innovation
February 8, 2024
Bio's View
John F. Crowley: ‘Biotechnology is another word for hope’
April 18, 2024
On April 18, 2024, the Biotechnology Innovation Organization (BIO) held the inaugural Agriculture & Environment Summit in Washington, D.C., bringing ...
Read More 5 reasons for investor optimism at BIO-Europe Spring 2024
March 29, 2024