New SBIR Foreign Disclosure Requirements: How Does It Impact Your Company?
New SBIR Foreign Disclosure Requirements: How Does It Impact Your Company?
September 19, 2023
Following reauthorization by Congress of the SBIR/STTR Programs in 2022, there are new rules companies should familiarize themselves with concerning required disclosures of foreign affiliations or relationships with foreign countries. In addition to mandating disclosures prior to receiving SBIR awards, companies must also regularly update NIH following any changes to a disclosure. View this webinar organized by the Biotechnology Innovation Organization (BIO) for a comprehensive discussion on how to effectively navigate these new rules as you apply for SBIR awards.
This webinar is co-hosted by the National Institutes of Health (NIH) Seed Office.
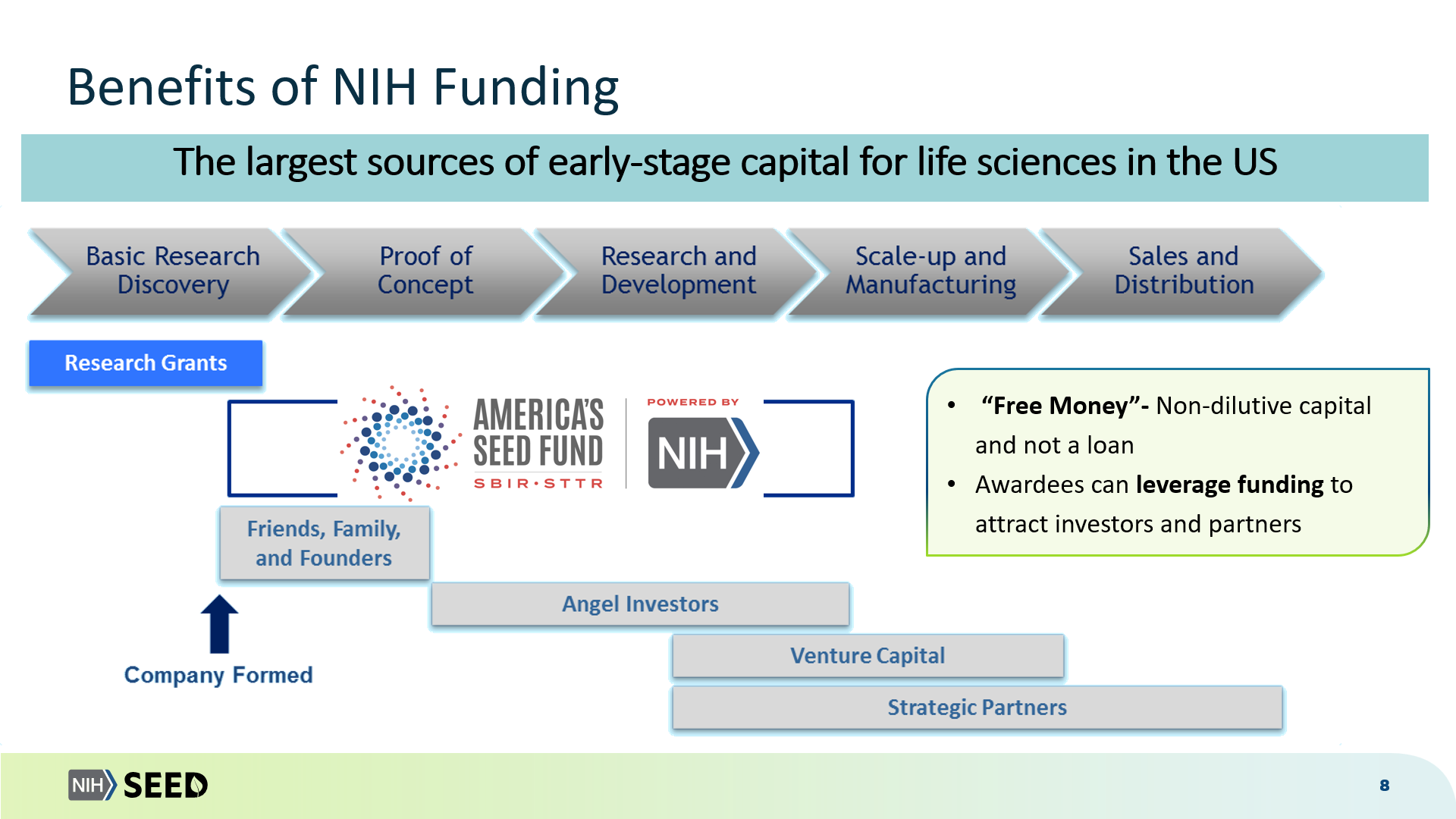
Webinar slides for New SBIR Foreign Disclosure Requirements and related SBIR information
Moderators & Speakers