Drug Development, Review & Lifecycle Management

Before a therapy is approved for delivery to patients a lot of hard work and research goes into demonstrating that the therapy is safe, effective, and high quality. We work to promote a regulatory environment that supports safe and efficient development and approval of therapies to treat patients afflicted with serious diseases, to delay the onset of these diseases, or to prevent them in the first place.
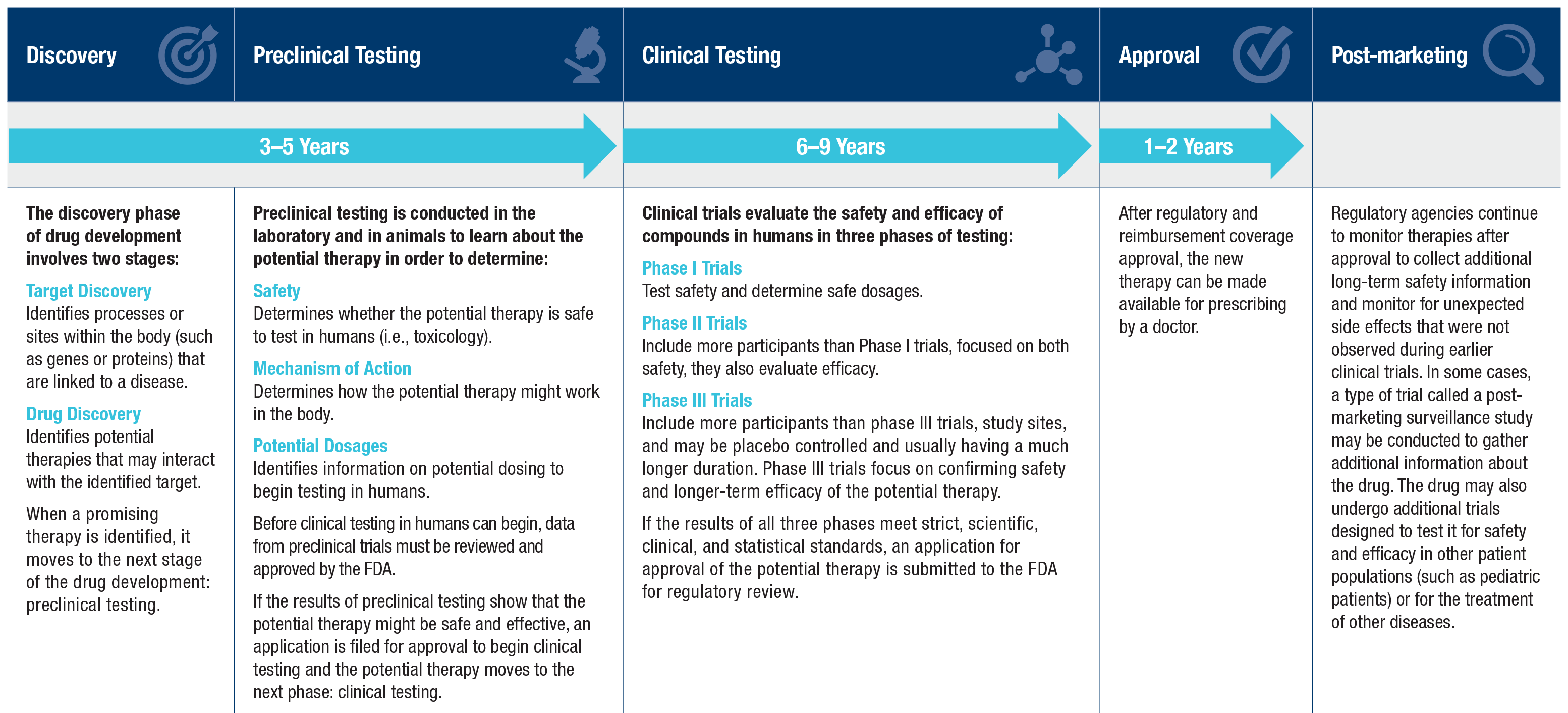
Drug Development and Clinical Trial Process
BIO White Paper on FDA’s Statement of Patient Experience
In September 2017, FDA implemented the Patient Experience Data table (PED Table) to be completed by reviewers and included in NDA/BLA review documents...

Become an Advocate for Biotechnology
Sign-up to join BIOAction, BIO's grassroots advocacy program. You will be notified about relevant policy issues where we need you to contact your lawmaker.
Biotechnology Innovation Organization
1201 New York Avenue NW, Suite 1300, Washington, DC, 20005 ・ 202-962-9200
© BIO 2025 All Rights Reserved