Learn how BIO Agriculture & Environment member companies are responding to the Coronavirus.
Here's a snapshot of how our industry is responding to the coronavirus.
Learn about how the biopharmaceutical industry is responding to the coronavirus.
To ensure people get safe and effective vaccines as quickly as possible, biotechnology companies have leveraged global partnerships and
innovative collaborations to reach patients all around the world.
A joint survey from BIO and BioCentury finds companies are seeking guidance and regulatory flexibility, while at the same time moving to remote monitoring and telemedicine for ongoing trials.
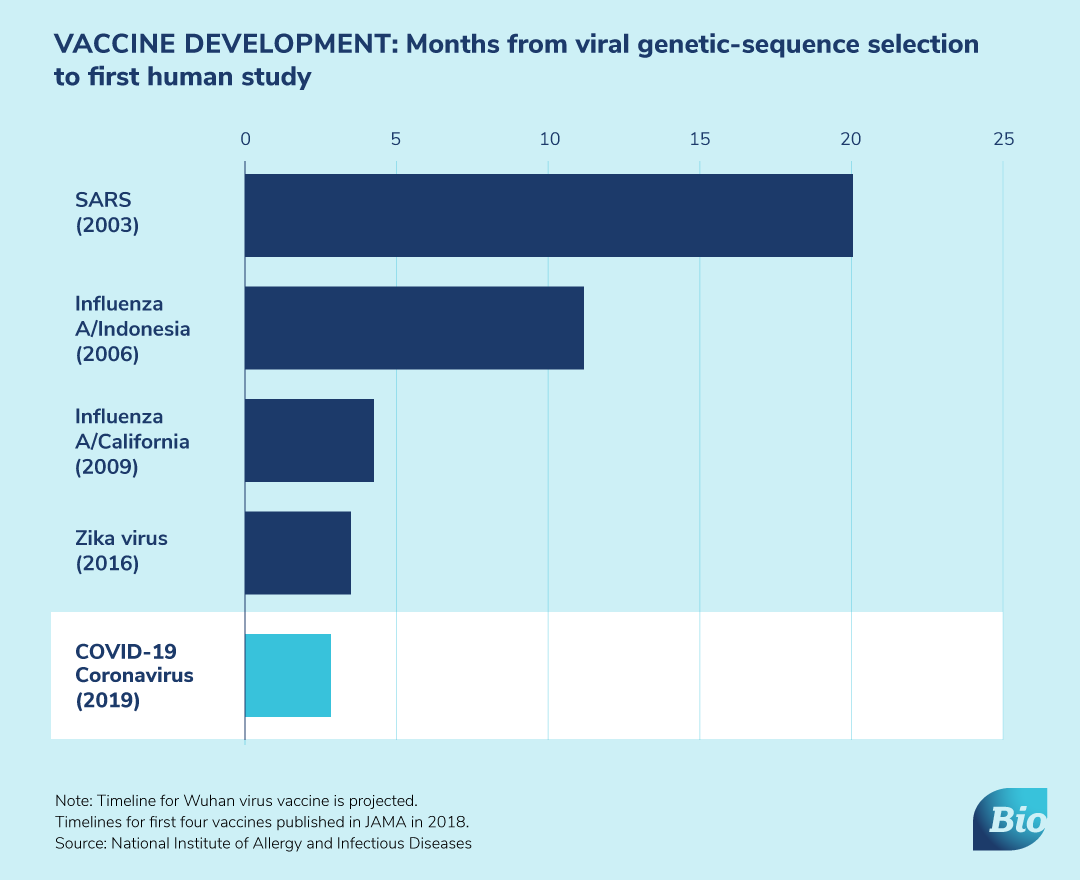
As the novel coronavirus continues to spread, scientists and researchers are working around the clock to identify diagnostics, therapeutics and vaccines to help protect individuals and communities across the globe.
On March 24-25, 2020, BIO virtually convened more than 500 leaders from across industry, government, academia, and NGOs to assess the state of the pandemic and accelerate medical countermeasure solutions for COVID-19.
Learn about how a COVID-19 vaccine will be procured and distributed during the public health emergency.
BIO virtually brought together 45 biotech innovators, government agencies, and leading academics to share information about their work to fight the coronavirus.
In addition, view the BIO Europe Spring Combating Coronavirus virtual session.

George Scangos, Ph.D., President and Chief Executive Officer of Vir Biotechnology, has been selected to lead the Biotechnology Innovation Organization's response to Coronavirus (COVID-19). Dr. Scangos explains what this role means and why collaboration across the private, public and NGO sectors is vital in combating Coronavirus.
Jim Greenwood in Morning Consult: The science is galloping forward, and collaboration between the public and private sectors is vital to combat the coronavirus. BIO is taking the lead to connect innovators with partners inside the federal government.