View this webinar to learn about the many exciting opportunities available at the 2025 BIO Asia-Taiwan International Conference & Exhibition.
This webinar covers:
- Overview of the event schedule
- Preview of conference sessions and programming
- Live demo of the BIO Partnering system, including new features such as Saved Search Notifications and Batch Responses for Meeting Requests, to enhance your business partnering experience
- Q&A with the speakers
Learn more and register for the event at

Join Thermo Fisher Scientific for an exclusive webinar introducing a transformative approach to biologics development and clinical scale-up. Their "Path to IND for Biologics" method accelerates the journey from DNA to IND/IMPD submission and first-in-human clinical trials in as few as 9 months. Dr. Elena Gontarz, a leading expert in the biologics industry, will guide you through this innovative process, leveraging AI/ML, transposase technology, and integrated solutions during the session: "Path to IND for Biologics: From DNA to First-in-Human in 9 Months." Following the presentation, Dr. Gontarz will answer audience questions and discuss how these novel strategies can streamline the development of complex biologics, such as Fc-fusion proteins and bispecific antibodies, driving them swiftly from development to commercialization.
Get a sneak peek on this novel platform
Terms and Conditions: Titer levels provided are estimates based on third party results and may vary depending on molecule type or other factors. Timeline from DNA to drug product and start of clinical trials for all path to IND for biologics options may vary depending on molecule type or other factors and are estimates to be finalized after third party cell line development dates are available and confirmed. 9-month timeline will incur additional risk.
Learn more about Dr. Elena Gontarz's expertise and background:

View this webinar for an in-depth exploration of today's complex global market landscape. This panel will address the challenges of launching new therapies in multiple countries, focusing on early decision points for risk mitigation and maximizing supply chain resiliency. Discover how Cencora's global capabilities and flexible solutions can strengthen supply chain resilience amidst evolving challenges.
Moderator: Sandra Anderson, Senior Vice President Commercialization, Cencora
Panelists:
- Glenn Pauly, Chief Commercial Officer, Alzheon
- Graham Goodrich, Chief Commercial Officer, Apnimed
- Leigh Shultz, PhD, Associate Vice President & International Head of Trade Channel Strategy & Enablement, Merck & Co.
- Kevin McDermott, Vice President International Commercialization, Cencora
- Tom Doyle, Head of U.S. Commercial Solutions, Cencora

More than 400,000 meetings are requested in the weeks leading up to the BIO International Convention. View this insightful webinar designed to help service providers optimize their approach to business development meetings at the BIO International Convention and turn their meeting requests into productive meetings. Learn how to leverage the BIO Partnering™ system effectively to connect with the right prospects, craft compelling meeting requests, and maximize your networking opportunities. Our panelists share proven strategies, best practices, and insider tips to help you enhance your business development efforts and drive meaningful connections in the life sciences industry.
This webinar is tailored to our attendees who represent service providers and vendors, but is open to anyone who believes they can benefit from hearing from experienced sales professionals.
More than $4B of deals between biopharma companies and AI specialists were announced last year. Similar to the massive quality leap from GPT-2 to GPT-4 and the record-breaking consumer adoption rates of AI tools, that same exponential progress and uptake is coming to biotech AI. 2024 may bring the first one-trillion-parameter protein language model. Companies are already using AI to program, as opposed to discover, antibodies. This level of opportunity and change brings with it urgency—— companies need to rethink from the ground up how their R&D should work to compete in a new era defined by the intersection of biology, data, and AI.
In this webinar, learn how companies are updating their approach to R&D, with a focus on:
- AI-Ready Data: Improving generation of high-quality biomedical data to support AI model building and model tuning
- AI-Enabled Scientists: Integrating the right models directly into scientists’ workflows for everyday use
- Scalability: Making performance, speed, privacy, and cost tradeoffs for deployment of AI, and connecting the wet and dry labs

Is this the first time you’re attending BIO International Convention? This webinar is for you! We’ll cover all the basics of where to go and what to do - plus, you’ll get some insider tips from the BIO team on this year’s exciting event.
If you haven't already registered, please learn more and register at convention.bio.org

BIO 2025 is around the corner—and with thousands of potential partners and limited time, preparation is everything. Whether you're a first-time attendee or a seasoned BIO veteran, this webinar will give you the tools and strategies to maximize your efforts—from communicating your objectives and optimizing your company profile to crafting compelling meeting requests and making the most of your time onsite.
Hear directly from industry panelists as they share insights and real-world advice drawn from their own BIO experiences. Learn what works, what doesn’t, and how to position yourself for success.
In this webinar, you'll learn:
- Best practices for building a standout BIO Partnering™ profile
- Tips for searching/identifying the right partners and prioritizing your outreach
- Do’s and don’ts for effective in-person meetings at BIO 2025
- What to expect onsite and how to follow up for long-term impact
- Key BIO Convention updates including networking opportunities and key delegations
Visit convention.bio.org to register or learn more.

BIO's new and improved partnering system — BIO Partnering™ — will open for the 2025 BIO International convention in early April. Join BIO's VP of Partnering, Mackensie Vernetti, for a webinar showcasing the new platform, complete with an in-depth live demo, as well as partnering tips and best practices. This webinar will provide the tools and guidance to maximize your experience at the event.
Key Takeaways:
- In-Depth Look at the Partnering System: Learn about features that streamline partnering discovery and make easy work of meeting management.
- What's New: See the newest system enhancements in action.
- Best Practices: Advice on how to search and find partners, send quality meeting requests and triage your messages.
- Quick Tips: Discover shortcuts and helpful tools to save you time.
Learn more about the BIO International Convention at

The BIO International Convention is the largest and most comprehensive event for biotechnology, representing the full ecosystem of biotech with 20,000 industry leaders from across the globe.
In a time of remarkable discovery and progress, the biotechnology industry stands at the forefront of a revolution changing how we live, heal, and care. View this webinar for a tour of the program's featured sessions, super sessions, and notable expert speakers, across the more than 140 sessions planned for BIO 2025 in Boston, June 16-19. Also, this webinar will be your first look at several new program session types and event surprises in progress, including a BIO Storytelling Stage, new evening reception venues, and an expanded main stage experience that will debut this year. The webinar includes advice on planning your calendar to maximize the value of attending.

Join Sydney Williams, BIO Senior Manager, Exhibit Sales & Systems, for a webinar to walk exhibitors through the tools needed to plan for the 2025 BIO International Convention.
This webinar gives our exhibitors the resources to have an impactful presence in Boston.
The following topics are discussed:
- Exhibitor resource center
- Exhibitor tools and resources
- Tips to maximize your event experience in Boston

The FDA has established the Split Real-Time Application Review (STAR) pilot program. The purpose of this program is to shorten the time from the date of a complete submission to the action date, in allowing earlier patient access to therapies that address an unmet medical need.
In this webinar, FDA staff will give an overview of the program, including the eligibility criteria for the pilot, and will answer your questions about participating in the program.

View this conversation highlighting how BIO member companies are leveraging biotechnology innovations to enable advances in climate adaptation, mitigation, and resilience. Hear how diverse feedstocks and manufacturing processes are targeting emission reduction breakthroughs in hard-to-reach sectors. Learn how regulation and policy can better incentivize innovation and be structured to support new and emerging technologies throughout research, development, demonstration, and deployment.

Following reauthorization by Congress of the SBIR/STTR Programs in 2022, there are new rules companies should familiarize themselves with concerning required disclosures of foreign affiliations or relationships with foreign countries. In addition to mandating disclosures prior to receiving SBIR awards, companies must also regularly update NIH following any changes to a disclosure. View this webinar organized by the Biotechnology Innovation Organization (BIO) for a comprehensive discussion on how to effectively navigate these new rules as you apply for SBIR awards.
This webinar is co-hosted by the National Institutes of Health (NIH) Seed Office.

With the majority of biological studies conducted in academic settings but nearly all innovative new drug clinical trials sponsored by industry, improving translational research success rates for commercialization is critical to bringing new treatments to patients. Surveys of academic tech transfer offices and the biopharma industry executives working on such alliances cite as obstacles two highly controllable factors: disagreement about alliance performance metrics and misalignment of deal terms priorities. This panel discusses best practices for removing such obstacles to accelerate more promising ideas into clinical development for patients.
BIO's updated 2023 report, "U.S. Biotechnology Translational Research: Partnership Models, Management Principles, and Best Practices," is available for download at the link below. The webinar also summarizes major findings from that BIO report as context for the panel discussion and audience Q&A.

The Cell and Gene Therapy (CGT) Science Series is a quarterly seminar series focused on scientific topics related to cell and gene therapy products. The CGT Science Series is intended to foster scientific exchange between the Biotechnology Innovation Organization (BIO), the American Society of Cell & Gene Therapy (ASGCT), and Center for Biologics Evaluation and Research (CBER) review staff on a variety of topics that span the CGT product lifecycle. The seminars are planned as 60-minute virtual webinars featuring a speaker from one of the three organizations. The CGT Science Series will enable a deep dive into a specific technical and/or scientific area. Topics in the series may include but are not limited to, nonclinical, CMC, clinical, or post-market phases of development related to CGT product lifecycle.
Moderator:
– Anne-Virginie Eggimann, Tessera
Opening Remarks:
– Cartier Esham, Senior Advisor, Biotechnology Innovation Organization (BIO)
Speaker:
– Jing Liao, Director, Vector Development and Operations, Alexion Pharmaceuticals

During this webinar, panelists discuss strategies for biotechnology companies to manage economic downturns and weakened investor interest in the biotech industry. Our expert panel of industry leaders share their insights and experiences on how to maintain momentum and emerge stronger during periods of economic uncertainty.
This webinar is designed to provide practical advice and actionable strategies that can be implemented immediately. The panel covers the following topics:
• Effective communication and investor relations strategies during economic downturns
• Actions biotechs can take to cut costs without sacrificing innovation and growth
• How to pitch to investors during weakened investor interest periods

U.S. Department of Justice (DOJ) statistics suggest that enforcement will be a top government priority in 2023, including for pharma/life sciences. Warning or untitled letters from the U.S. Food and Drug Administration (FDA) will serve as ammunition for DOJ cases. Statements about product safety will be heavily scrutinized.
Do you know what to watch out for? Has your company trained its employees and marketing agencies on how to appropriately implement the requirements for promotion? What activities require extra vigilance? The prior downturn in enforcement can be linked to the development of a response to COVID-19, which diverted government resources across the board. Now trends suggest the FDA, DOJ, and Office of Inspector General (OIG) will return to active healthcare enforcement in 2023 for violations of the Food Drug and Cosmetic Act (FDCA), False Claims Act and the Anti-Kickback Statute in part attributable to the goal of obtaining additional financial recoveries.
This webinar hosted by the Biotechnology Innovation Organization (BIO) has these learning objectives:
—Understand the current trends for government enforcement
—Dissect the compliance implications of increased enforcement on the industry
—Identify specific cases that are clear examples for company concern
—Isolate the key principles for avoiding violations
ON-DEMAND
COMMUNICATIONS COMPLIANCE COURSES
FOR PROFESSIONAL DEVELOPMENT
Special Discount
Buy 2 course registrations, Get 1 FREE
through May 15, 2023
in honor of this webinar!
The Center for Communication Compliance (CCC) helps biopharmaceutical companies achieve efficient, compliant innovation in a digital world through its proprietary on-demand business solutions in education, technology, and change management.
The CCC eLearning System has set an unmatched standard for confirming regulatory competency through engaging mastery tests and expert education content across more than a dozen course options.
SEE COURSES LIST

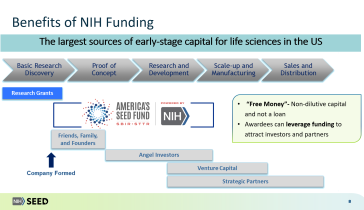
Following reauthorization by Congress of the SBIR/STTR Programs in 2022, there are new rules companies should familiarize themselves with concerning required disclosures of foreign affiliations or relationships with foreign countries. In addition to mandating disclosures prior to receiving SBIR awards, companies must also regularly update NIH following any changes to a disclosure. View this webinar organized by the Biotechnology Innovation Organization (BIO) for a comprehensive discussion on how to effectively navigate these new rules as you apply for SBIR awards.
This webinar is co-hosted by the National Institutes of Health (NIH) Seed Office.

With the majority of biological studies conducted in academic settings but nearly all innovative new drug clinical trials sponsored by industry, improving translational research success rates for commercialization is critical to bringing new treatments to patients. Surveys of academic tech transfer offices and the biopharma industry executives working on such alliances cite as obstacles two highly controllable factors: disagreement about alliance performance metrics and misalignment of deal terms priorities. This panel discusses best practices for removing such obstacles to accelerate more promising ideas into clinical development for patients.
BIO's updated 2023 report, "U.S. Biotechnology Translational Research: Partnership Models, Management Principles, and Best Practices," is available for download at the link below. The webinar also summarizes major findings from that BIO report as context for the panel discussion and audience Q&A.

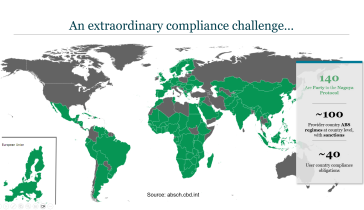
View this important webinar for those invested in the broader biotech industry, "Understanding the Business Implications of Global Access and Benefit Sharing Policies." Tailored for members of BIO and a diverse community of stakeholders, this high-level session focuses on understanding the impact of recent developments in the global policy arena that will affect industry’s access to vital biologic materials and information. These policies have implications for the way industry will need to share the potential benefits that flow from their use of these physical and digital resources worldwide. Through interactive discussions led by seasoned experts across the Biotech ecosystem, explore the intricate relationship between national and global concerns, while uncovering the potential implications for your company. This webinar will equip you with the knowledge and insights essential to thriving in an ever-evolving global biotech landscape for an empowering dialogue that promises to shape the future of biotechnology.

The BIO Investor Forum is back! Join us at the Hilton San Francisco Union Square from October 17-18 for two days of thought-leadership, partnering, and insight into investment trends and the priorities of venture-stage growth and emerging public biotech companies.
View this webinar to learn more about what’s happening this year, including a half-day pre-conference event, the BIO Seed-Stage Spotlight on Tech Transfer with 34 fast pitches from start-ups outlining their drug development programs. This webinar will detail what the BIO One-on-One Partnering™ system can do for your business goals and how to use the system's powerful features. Explore the program, networking opportunities, partnering system fundamentals, gain insight into best practices for partnering, and more.
For more information and to register for the BIO Investor Forum, please visit: https://www.bio.org/events/bio-investor-forum
Register now for the conference to get access to the partnering system and to request your meetings.

The Cell and Gene Therapy (CGT) Science Series is a quarterly seminar series focused on scientific topics related to cell and gene therapy products. The CGT Science Series is intended to foster scientific exchange between the Biotechnology Innovation Organization (BIO), the American Society of Cell & Gene Therapy (ASGCT), and Center for Biologics Evaluation and Research (CBER) review staff on a variety of topics that span the CGT product lifecycle. The seminars are planned as 60-minute virtual webinars featuring a speaker from one of the three organizations. The CGT Science Series will enable a deep dive into a specific technical and/or scientific area. Topics in the series may include but are not limited to, nonclinical, CMC, clinical, or post-market phases of development related to CGT product lifecycle.
Moderator:
– Anne-Virginie Eggimann, Tessera
Opening Remarks:
– Cartier Esham, Senior Advisor, Biotechnology Innovation Organization (BIO)
Speaker:
– Jing Liao, Director, Vector Development and Operations, Alexion Pharmaceuticals

View this webinar to learn about the many exciting opportunities available at the 2023 BIO Asia-Taiwan International Conference & Exhibition. This webinar covers:
- Overview of the event schedule
- Preview of conference sessions and programming
- Live demo of the BIO One-on-One Partnering system, including new features such as Optional Meeting Participants, to enhance your business partnering experience
- Q&A with the speakers
Conference details at this link.

This webinar is for BIO 2023 attendees who are familiar with the BIO One-on-One Partnering™ system and want to use the platform more effectively. View this advanced partnering webinar—led by BIO’s Director of Partnering—to learn tips & tricks, commonly overlooked yet powerful features that will help you better identify companies of interest, and more. You can leverage these insights and features to take your partnering experience to the next level in Boston at this year's BIO International Convention.
Learn more and register for BIO 2023 at this link.

During this webinar, panelists discuss strategies for biotechnology companies to manage economic downturns and weakened investor interest in the biotech industry. Our expert panel of industry leaders share their insights and experiences on how to maintain momentum and emerge stronger during periods of economic uncertainty.
This webinar is designed to provide practical advice and actionable strategies that can be implemented immediately. The panel covers the following topics:
• Effective communication and investor relations strategies during economic downturns
• Actions biotechs can take to cut costs without sacrificing innovation and growth
• How to pitch to investors during weakened investor interest periods

U.S. Department of Justice (DOJ) statistics suggest that enforcement will be a top government priority in 2023, including for pharma/life sciences. Warning or untitled letters from the U.S. Food and Drug Administration (FDA) will serve as ammunition for DOJ cases. Statements about product safety will be heavily scrutinized.
Do you know what to watch out for? Has your company trained its employees and marketing agencies on how to appropriately implement the requirements for promotion? What activities require extra vigilance? The prior downturn in enforcement can be linked to the development of a response to COVID-19, which diverted government resources across the board. Now trends suggest the FDA, DOJ, and Office of Inspector General (OIG) will return to active healthcare enforcement in 2023 for violations of the Food Drug and Cosmetic Act (FDCA), False Claims Act and the Anti-Kickback Statute in part attributable to the goal of obtaining additional financial recoveries.
This webinar hosted by the Biotechnology Innovation Organization (BIO) has these learning objectives:
—Understand the current trends for government enforcement
—Dissect the compliance implications of increased enforcement on the industry
—Identify specific cases that are clear examples for company concern
—Isolate the key principles for avoiding violations
ON-DEMAND
COMMUNICATIONS COMPLIANCE COURSES
FOR PROFESSIONAL DEVELOPMENT
Special Discount
Buy 2 course registrations, Get 1 FREE
through May 15, 2023
in honor of this webinar!
The Center for Communication Compliance (CCC) helps biopharmaceutical companies achieve efficient, compliant innovation in a digital world through its proprietary on-demand business solutions in education, technology, and change management.
The CCC eLearning System has set an unmatched standard for confirming regulatory competency through engaging mastery tests and expert education content across more than a dozen course options.
SEE COURSES LIST